Can Saccharomyces boulardii lead the next generation of engineered probiotics?
By David Sáez Moreno
Why Yeast?
When we think of probiotics, we normally think of bacteria: Lactobacillus, Lactococcus or other lactic acid bacteria, and we normally forget that there is also a eukaryotic probiotic!
Saccharomyces boulardii is a probiotic yeast that is on the market and is used as standard of care for preventing traveler´s diarrhea (if you like backpacking, you probably know what I am talking about) or to mitigate dysbiosis after antibiotic treatment. There are now 45 completed clinical trials including the use of S. boulardii, and several S. boulardii-based probiotic products in the market, and a lot of scientific interest in recent years. See in Fig.1, how the number of publications referring to it is increasing year by year.
S. boulardii has shown antimicrobial properties, an ability to neutralize toxins, direct binding to pathogens, production of Short Chain Fatty Acids, stimulation of the immune system, of mucin production, antioxidant effects… and has one main advantage over lactic acid bacteria, which is the fact that it is not affected by antibiotics.
So, while it is known that it promotes human health, and we understand now a lot of how this happens, there are still gaps and some limitations. S. boulardii is not naturally part of human gut microbiota, competes with the microbiome, and normally shows slow residence times in the gut. So, here is where genetic engineering enters.
Figure 1. Number of scientific papers published per year on Saccharomyces boulardii, according to PubMed®. Total number of publications contemplating S. boulardii in the last 42 years (1982–2024). Data were obtained from PubMed® using the queries: “Saccharomyces boulardii” and filtered by year (1982 to 2024). https://pubmed.ncbi.nlm.nih.gov Accessed in April 2025. Figure from (1).
Advantages of yeast for genetic engineering.
When it comes to genetic engineering, there is a reason why I like yeast. Contrary to most bacteria, it admits very large chunks of DNA. If you have a large DNA fragment to clone and bacteria don’t seem to want it, you can put it into yeast (most times 😉). There are whole pathways introduced into yeast, and it can produce more complex proteins, because of its ability to glycosylate proteins. Imagine complex molecules coming from humans, plants or insects, being produced in yeast: human cytokines, therapeutic peptides, and even vaccine antigens.
Genetic engineering offers the advantage of rationally designing the delivery of molecules of interest to the gut, to basically improve the health-benefit effects. It also provides a way for us to improve and correct the limitations of the probiotic, for example, its low retention time in the gut.
Has this been done before?
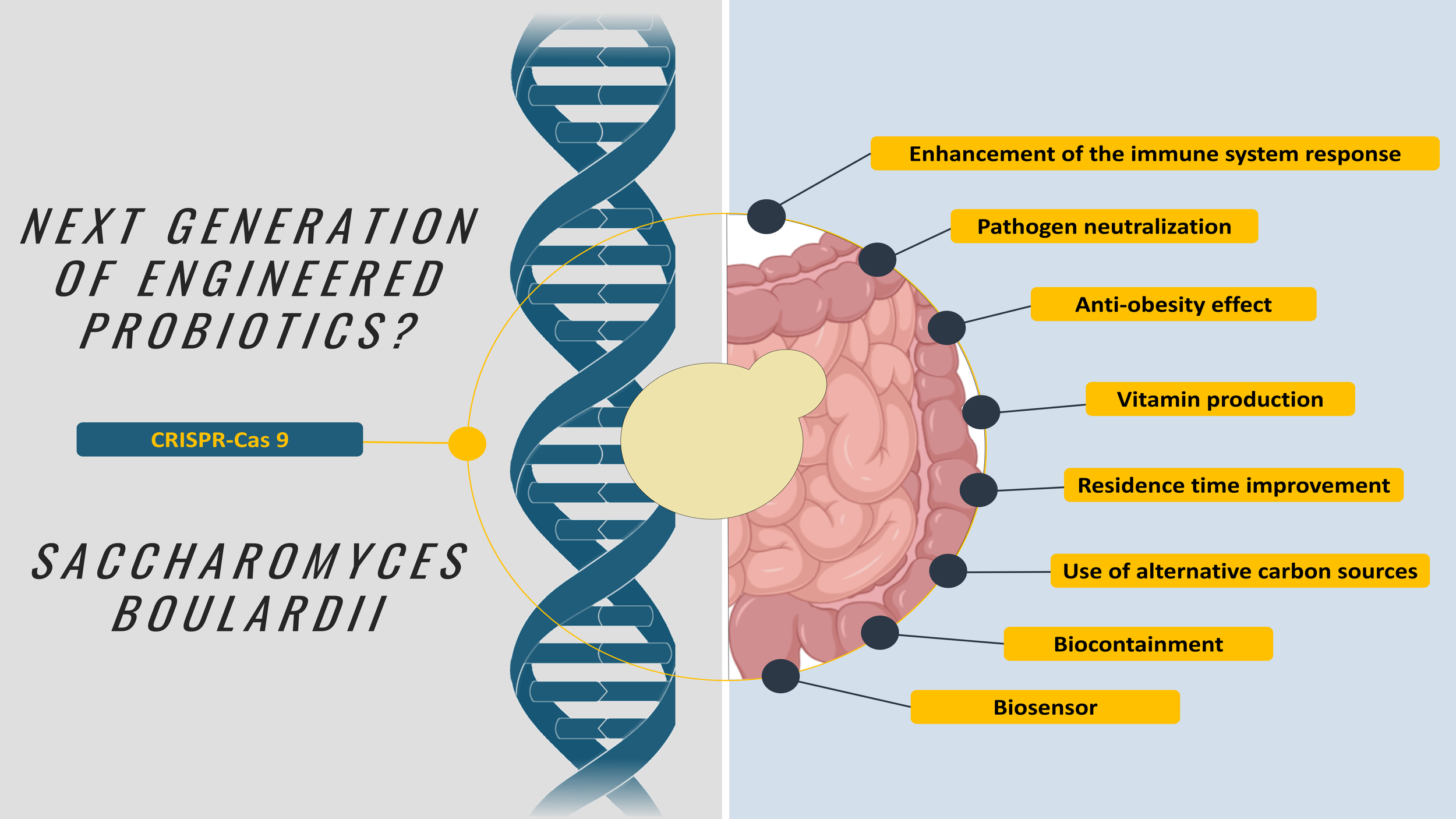
Yes! S. boulardii has been engineered towards many different applications already: to enhance immune system response, to improve its pathogen neutralization ability, to improve its retention time in the gut, to enhance anti-obesity effects, to produce vitamin precursors, for biocontainment, for the use of alternative carbon sources, for use as biosensor…
There have been many attempts to do this, increasing its capacity to neutralize pathogens, use alternative carbon sources, to produce vitamins, to enhance the immune system response… For example, modified strains have been engineered to express interleukin-10 to combat inflammatory bowel disease (2) or to produce Exendin, a glucagon-like peptide receptor agonist used in diabetes treatment, effective in preclinical models (3).
Figure 2. Overview from the genetic engineering applications of S. boulardii (38 studies total published between the years 2013 and 2025). Figure from (1).
And here comes the best part: the tools!
Interestingly, S. boulardii is genetically 99% identical to S. cerevisiae. However, if you think of the 12 megabases that baker´s yeast DNA contains, a difference of 1% can be huge: 120,000 bp, which is probably why S. boulardii is not used for beer-brewing. But, this difference is also small enough to take advantage of the wide number of genetic engineering tools that have been developed for the model organism S. cerevisiae. You name it: CRISPR-Cas, Cre-LoxP, transposons of yeast, high copy or episomal plasmids, yeast centromeric plasmids, yeast artificial chromosomes, yeast-integrating plasmids…
Advances in genetic engineering have led to the development of CRISPR-Cas9, which enables the engineering of S. boulardii. This approach results in a markerless and scarless chromosomal insertion, eliminating the need for antibiotics to maintain the modifications and leaving no marks in the genome. This not only reduces the cost of production but also facilitates its regulatory path. And if you think of the fact that S. boulardii is naturally not affected by antibiotics, it could be part of the solution for many gut diseases produced by bacteria, such as C. difficile.
Next steps.
On the personal side, during my PhD at the University of Minho, part of my work focused on using CRISPR-Cas 9 to introduce antibacterial molecules, endolysins, into S. boulardii, to deliver them to the gut. I hope I can share more on that soon!
As you can imagine, once genetic engineering enters the equation, the possibilities are almost endless: can we reduce intestinal tumours by enhancing the immune response? Can we deliver antibacterial agents? What about sensing molecules that predict disease? Delivering molecules directly to the gut can also minimise the side effects, in combination with the already health-promoting properties of the probiotic.
Of course, the next step is to proceed with clinical trials and demonstrate that the proof of concept work is published, and actually works when translated into the clinic. There are in fact a couple of genetically modified (bacterial) products already in the US market (see Zbiotics), which means the door is open!
Figure from (1).
References
1. Carvalho, J.P., Sáez Moreno, D., & Domingues, L., 2025. Genetic engineering of Saccharomyces boulardii: Tools, strategies and advances for enhanced probiotic and therapeutic applications. Biotechnology Advances, 84, 108663. ttps://doi.org/10.1016/j.biotechadv.2025.108663
2. Liu, J.J., Kong, I.I., Zhang, G.C., Jayakody, L.N., Kim, H., Xia, P.F., Kwak, S., Sung, B.H., Sohn, J.H., Walukiewicz, H.E., Rao, C.V., Jin, Y.S., 2016. Metabolic engineering of probiotic Saccharomyces boulardii. Appl. Environ. Microbiol. 82, 2280–2287. https://doi.org/10.1128/AEM.00057-16.
3. Hedin, K.A., Zhang, H., Kruse, V., Rees, V.E., B¨ ackhed, F., Greiner, T.U., Vazquez- Uribe, R., Sommer, M.O.A., 2023. Cold exposure and Oral delivery of GLP-1R agonists by an engineered probiotic yeast strain have Antiobesity effects in mice. ACS Synth. Biol. 12, 3433–3442. https://doi.org/10.1021/acssynbio.3c00455.